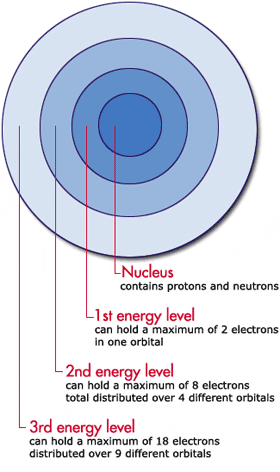
The first energy level closest to the nucleus has the least amount of energy. In ascending order the shell letters are K L M.

How Many Electrons Can Fit In The Second Energy Level At Level
At the next energy level there are four orbitals.
. If you are given only value of n you can easily calculate maximum number of electrons per every energy level every value of n using 2n2. In the first energy level there can only be two electrons. The letter designations for the first four sublevels with the maximum number of electrons that can be accommodated in each sublevel are.
The first shell is just 1S which can hold 2. The energy levels are typically referred to by their shell number rather than their energy level. Thus the first energy level holds 2 12 2 electrons while the second holds 2 22 8 electrons.
A set of three p orbitals called the p sublevel can hold a maximum of six electrons. The energy of an electron when it is far away from the influence of the nucleus is taken as zero. Energy levels are a little like the steps of a staircase.
These numbers are quantum numbers. Where R H is called Rydberg constant whose value is 21810 18 J. The maximum number of electrons possible in the first four energy levels are.
All levels except the first have p orbitals. Principal quantum number of an electron existing in such a stationary state is taken as n. Energy levels also called electron shells are fixed distances from the nucleus of an atom where electrons may be found.
At the third level there is a set of five d orbitals with complicated shapes and names as well as the 3s and 3p orbitals 3px 3py 3pz. If by first level you mean the 1s level in a hydrogen or hydrogen-like atom the answer is two. After that each energy level can have up to eight electrons.
E n R h 1 n 2. The maximum number of electrons found on energy levels one through six are two eight 18 32 50 and 72. In this sense the third shell can hold a total of 18 electrons.
Third energy level n 3. Each principal energy level above the first contains one s orbital and three p orbitals. Following the formula the third energy level can contain 18 electrons the fourth energy.
All the energy levels are denoted by integers eg. A 2s 2p1 2p2 and a 2p3. The main energy level that can hold only two electrons.
When there are no excited electrons energy levels fill up innermost to outermost. Number of orbitals 12 1 1s. How many electrons can be held on the 1st energy level.
How many electrons are in 4f orbital. At the lowest energy level the one closest to the atomic center there is a single 1s orbital that can hold 2 electrons. There are a total of 10 electrons.
The electrons in an atom can exist between energy levels. Electrons are tiny negatively charged particles in an atom that move around the positive nucleus at the center. The maximum number of electrons that an energy level can hold is determined from the formula 2n2 equals the total number where n is the energy level.
Each shell must be full before the next starts to fill. So the third shell can be considered to hold 8 or 18 electrons but in total the third shell can hold 18 electrons. Each of these orbitals can hold 2 electrons so a total of 8.
I cant think of any atom with a level equal to or below 1s but in molecular orbitals things can get very weird so I cannot say with certainty that there is no level lower than. Answer 1 of 6. Second energy level n 2.
This model breaks down. Of orbitals n2 22 4 1s 2px 2py 2pz. 32 Using the above you can work out the maximum number of electrons that can occupy a shell is 2n 2.
The second shell is 2S 2P which can hold 8 total 26 The third shell is 3S 3P 3D which can hold 18 total 2610 The fourth shell is. In this sense the third shell can hold 8 electrons. Electrons are placed into available shells starting with the lowest energy level.
N1 or 2 3 and so on. There are 2 in the first energy level 6 in the second and 2 in the third. Therefore the second level can contain a maximum of eight electrons - that is two in the s orbital and 6 in the three p orbitals.
Electrons can move up and down energy levels by gaining or losing energy. The number of orbitals found in the second energy level will be equal to. The formula for determining the number of electrons is two multiplied by n squared or 2n2.
The total number of orbitals that can exist at the second main energy level is. Answer 1 of 3. The range of the quantum number can fluctuate between the lowest energy level to the highest energy level.
Shells are not full if they do not hold the maximum. Maximum number of orbitals in an energy level n2. This means that the number of orbitals found in the first energy level will be equal to.
This means that before the second shell can begin being filled it must have a full first shell. Such kind of hydrogen atom is called an ionized hydrogen atom. In addition to s and p orbitals there are two other sets of orbitals which become available for electrons to inhabit at higher energy levels.
The first energy level K contains 2 electrons second energy level 8 third energy level 18 and fourth energy level has 32.

Interactives The Periodic Table It S Elementary For A Mad Scientist
How Many Electrons Can The Third Energy Level Hold At Level


0 Comments